Salt
WHAT EXACTLY IS SODIUM AND ITS USES?
Sodium (Na) is a light alkali metal belonging to the third period of the periodic table. It is an extremely reactive material and could even react with air itself. It has a higher reactivity and is placed into the category of highly reactive metals in the chart depicting the activity series of metals. It plays an important role in the functioning of the human body as well as the industries. Sodium is used in industries in appliances like heat exchangers, nuclear reactors, water softeners and in food industries as a common salt (NaCl). One of the most common uses of sodium is its consumption in the form of common salt when it’s combined with chlorine. Since seawater is naturally saline in nature and contains a huge amount of salt, it is one the largest sources of salt on earth.
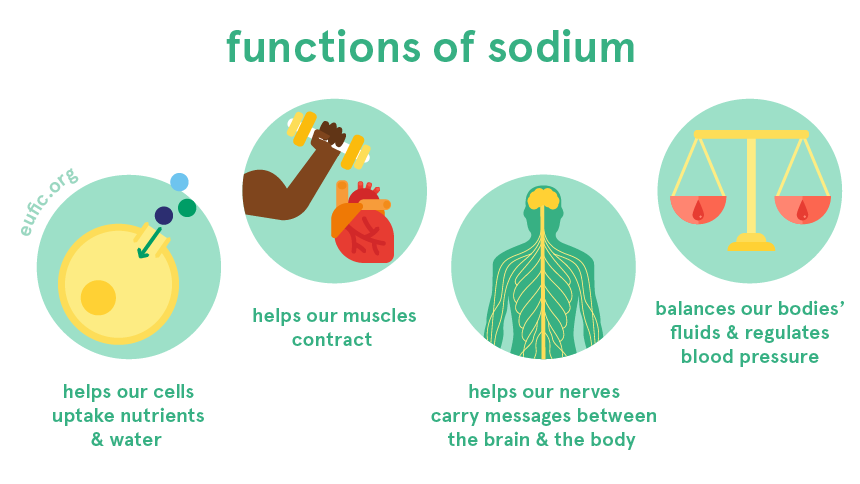
The figure shows the common uses of sodium in the functioning of the human body
HOW DO WE EXTRACT SALT AND OTHER MINERALS FROM SEAWATER?
Various methods have been investigated to extract salt from seawater. The most common methods proposed for obtaining salt are solar/vacuum evaporation, electrodialysis, membrane distillation/crystallization, and adsorption/desorption/crystallization. Minerals which are commonly mined using these methods include Sodium Chloride (NaCl), Magnesium Sulfate (MgSO4), Magnesium Hydroxide [Mg (OH)2], Calcium Carbonate (CaCO3), and Bromine (Br). These minerals have abundant uses in food, glass, steel, paper, agricultural, pharmaceutical, electrochemical, nuclear and construction industries.
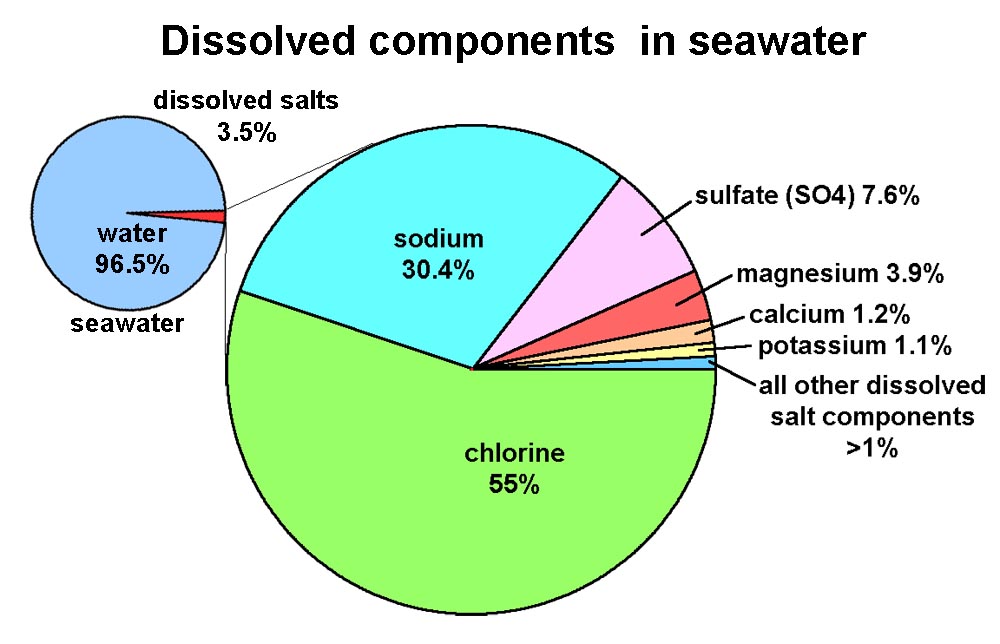
The figure shows the common elements like metals which are present in seawater
WHAT IS THE MECHANISM BEHIND THE WORKING OF THESE METHODS?
Solar/Vacuum Evaporation- The method for recovering minerals like salt from seawater and seawater desalination involves natural evaporation of water using the sun’s energy and leaving behind a concentrated salt solution. This leads to crystallization of salts when they become saturated. Crystallization is the process of formation of solids by minimizing the overall energy of the system by binding the atoms and molecules together in a well-defined manner. In this manner, it is used as a separation technique to separate solids from a solution. This method is particularly useful in arid regions receiving higher amounts of sunlight and higher evaporation rates. It is preferable that the evaporation vessels/ponds be shallow with a larger surface area for effective evaporation. Increasing the salt density from an initial value of less than 5% to a value greater than 25% could cause NaCl to reach its saturation point and crystallize.
One method employed in salt farming in Goa, India involved the use of three distinct pans known as the reservoir, evaporator and crystallizer pans respectively. The reservoir pan is where the seawater was stored initially. This reservoir pan was interconnected with several evaporation pans which in turn were connected to the crystallization pans. The waters in the pan were released from one pan to the other when certain salinity values were reached. Minerals like CaCO3 start to precipitate in the reservoir pan and CaSO4 crystallizes in the form of gypsum in the evaporator pan. Finally, NaCl precipitates in the crystallization pan. These pans have a size ranging from 8-46 cm in depth which is efficient on a small scale. The only limitation this method has is that solar evaporation ponds require a large surface area and may be susceptible to land pollution. If these two conditions are not a problem, then this is the easiest method to be implemented.
A colorful depiction of solar evaporation ponds used to obtain elements like NaCl and lithium. The most consequential drawback of this method is the enormous land area required and environmental pollution caused by leaching of the metals into the groundwater table. Moreover, the final product obtained in the form of lithium hydroxide (LiOH) is not deemed to be suitable for use in lithium-ion batteries. The figure represents the lithium field of Ganfeng Lithium Co, the world’s largest lithium producer. Recently, Tesla secured the lithium supply contract from this company, for the element to be used in electric vehicles (EVs)
Electrodialysis (ED)- This is an electro membrane process which involves extracting and concentrating ions in a solution by means of migration of ions under the influence of an electric field, through anion and cation selective semi-permeable membranes. Anion selective membranes allow only anions to pass through and cation selective membranes allow cations to pass through. These membranes are typically arranged in an alternating fashion to form a structure known as the cell, which consists of volumes of two adjacent membranes. The ion migration causes concentration in one cell and depletion in the other cell. This results in desalting and salting in specific areas of the cell. Selective monovalent cation and anion permeable membranes can extract monovalent ions like Na+
and Cl- from divalent ions like Mg2+, Ca2+ and SO4 2-. Monovalent ions are those ions which have a charge of magnitude 1. Examples of monovalent ions are Na+ and Cl-.
Because of greater rejection of divalent ions, the purity of NaCl produced was higher than the solar evaporation method described before. NaCl was obtained by the process of crystallization by evaporation following the rejection of divalent ions. Moreover, the NaCl depleted solution had 5 times more concentration of magnesium ions (Mg2+). Therefore, magnesium could also be extracted by precipitating Mg2+ as Mg (OH)2 by adding a base like NaOH. Chemical precipitation like crystallization is also a process of converting a solution into solid by converting a substance into an insoluble form or by making a supersaturated solution. The electrodialysis process uses electricity as the energy source therefore electrical energy is needed to be considered into the operational cost. Some limitations of this method may include scaling of membranes due to carbonate (CO3 2-) and sulfate (SO4 2-) precipitation on the membranes. Therefore, pre-treatment of the feed is required to prevent acidification by formation of calcium carbonate on the membranes. Membranes with anti-fouling properties are currently under development to counteract these problems.
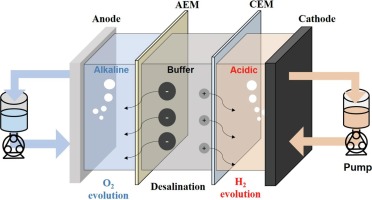
A schematic diagram of semi-permeable ion exchange membranes used for water treatment and desalination. These membranes have preferential permeation of certain ionic species and are classified into cation exchange membranes and anion exchange membranes. A common type of this membrane is obtained in the name of FORBLUE SELEMION from a tech company named AGC
Membrane Distillation Crystallization (MDC)- This method involves a thermally driven
operation where a hydrophobic microporous membrane separates pure water produced as
distillate from the brine solution. Hydrophobic materials are defined as those materials which have a very low affinity to water and thus do not dissolve in water. This hydrophobic nature prevents the penetration of water into the pores of the microporous membrane, creating a vapor-liquid interface at each pore entrance. During this process, the water evaporates at the membrane interface at the warm side, diffuses through the pores, and condenses on the other colder side. This process maintains a tight supersaturation of salts while the process of crystallization takes place in a circulating crystallizer. The benefit of this method is in creating and maintaining a supersaturated solution where crystal nucleation (birth of new crystals) and growth can occur easily. Crystals are formed as a by-product. Also, one can control the supersaturation levels inside a brine solution. This, in turn, produces higher quality crystals compared to techniques like cooling and evaporative crystallization. The apparatus used for this method involves the application of a distillate tank and a crystallizer tank, each serving their own purpose.
During laboratory experiments conducted by researchers using the above apparatus, high purity NaCl and MgSO4.7H2O (Epsom salt) have been effectively extracted. These crystals obtained constituted a qualitatively better product since membrane crystallizers are characterized by a unidirectional axial flux of the crystallizing solution through the membrane which reduces the mechanical stress and improves the homogeneity of the crystallizing solution. Consequently, the crystals obtained possessed good structural properties and narrow distribution. The only limitation which this method has is that it is able to extract only those salts having high concentrations in seawater and brines. However, since we want to extract NaCl and CaCO3 which are already present in abundant amounts, it should not be a major problem implementing this method.
Membrane distillation is a thermal separation strategy and is considered one of the most attractive technologies for seawater desalination purposes. This technology could be operated at lower hydraulic pressures, promotes high rejection capacity of non-volatile components, and leaves behind a smaller footprint. Due to the operational properties of both MD and crystallization, the integration/combination of MD with the crystallization process (MDC) has high potential for the treatment of high salinity solutions without additional post-treatment.
Adsorption/Desorption Process- Adsorption is the process of separation of a substance from one phase by its accumulation at the surface of another phase. The adsorbing phase is called the adsorbent, whereas the material concentrated at the surface of that phase is called an adsorbate. It is essentially a surface phenomenon which could also be applied to extract those minerals which occur at lower concentrations. The adsorbent typically needs to have a higher selectivity towards the mineral of interest in the presence of other minerals present in seawater brine. Following this, the mineral is easily desorbed using minimum volume and concentration of the desorbent and precipitated to crystallize the mineral. In case other minerals are present in the desorbed solution, they must be qualitatively separated using precipitation or other adsorbents selective to them to prevent their interference with crystallization. Also, extraction of minerals from seawater brines is much easier than the extraction of minerals from seawater since they are 2-3 times more concentrated in the brine. Some common adsorbents which could be used are inorganic compounds, organic polymeric ion exchange resins, chelating (agents which have a ringed structure) resins, and nanomaterials.
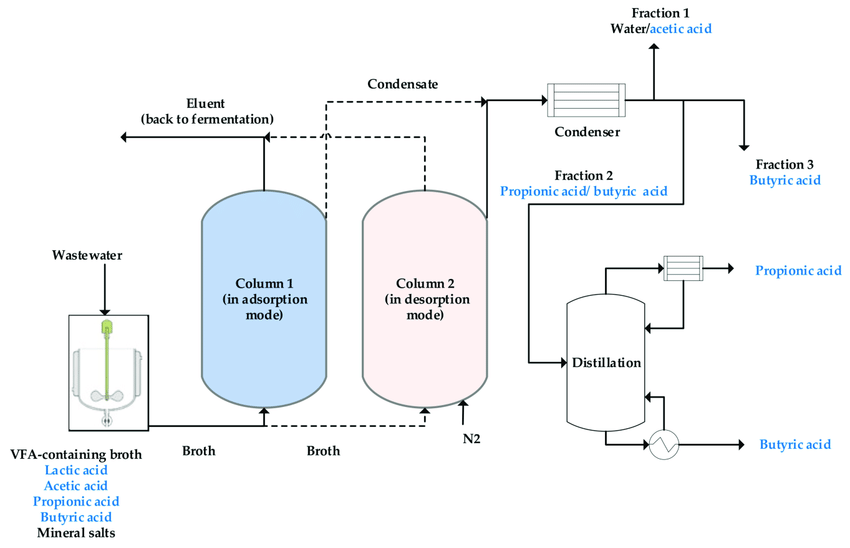
Illustration of the general process of adsorption and desorption to obtain a pure mineral
This process could be effectively used to extract minerals like Lithium (Li), Uranium (U), Strontium (Sr), and Rubidium (Rb). For lithium, H-form of ɑ-MnO2 has proven to have the highest adsorption capacity for Li from seawater. The only limitation to this method is that there needs to be thorough research on desorbing agents needed in obtaining the adsorbed mineral.
