What Is Brine?
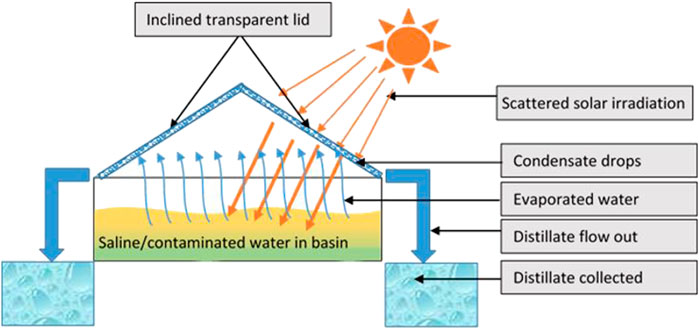
Brine is defined as a typical hypersaline concentrate which is the leftover product obtained from evaporating seawater. It is usually of limited purposes and is typically discharged in seawater or injected in underground deep wells during the process of elimination.
Currently, most of the waste brines, especially brines produced in seawater desalination plants, are directly discharged into adjacent open water bodies. This is marine pollution as doing so greatly increases the salinity of the waters and wreaks havoc on fragile aquatic ecosystems. Therefore, methods of proper dispersal of brine are needed to be devised for safety and efficiency of brine disposal.

Currently, brine production stands at a massive 141.5 million m3/day. This value is approximately 50% greater than the total volume of desalinated seawater produced every day.
Composition
As mentioned above, brine is the highly concentrated saline water obtained in the last phase of the desalination process. Saltwater’s composition is made of multiple valuable elements.
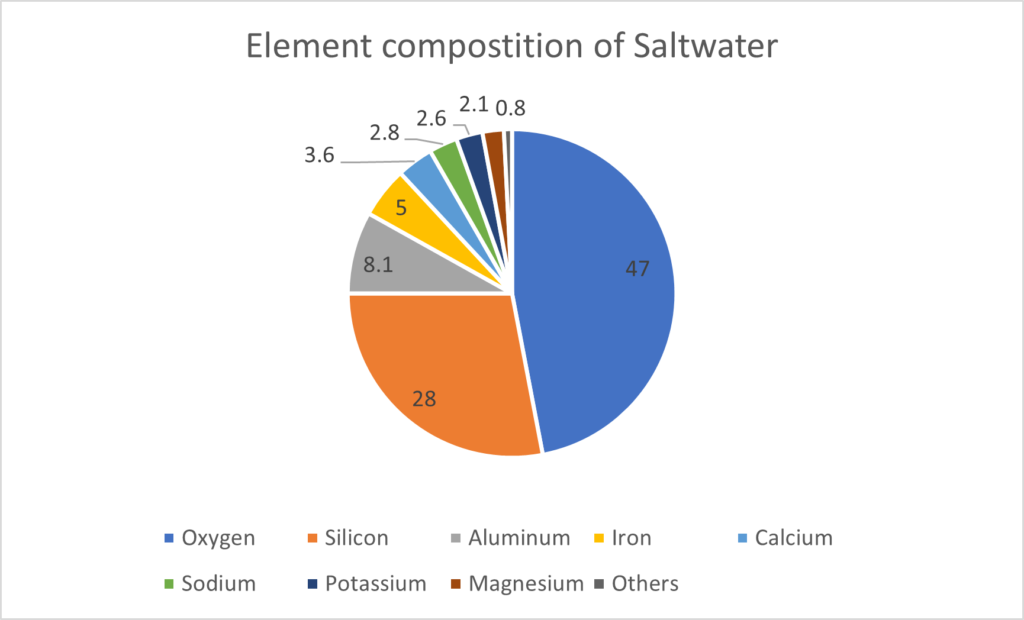
| Rare Earth Metals: | Cerium, Scandium |
| Precious Metals: | Palladium, Platinum |
| Radioactive Metals: | Radium, Uranium |
| Alkaline Metals: | Lithium, Magnesium |
| Nutrients: | Ammonia, Nitrate, Phosphate |
| Trace Organic Chemicals: | Pesticides, Personal Care and Pharmaceutical Products, Effluent organic matter and pathogens |
Disposal of brine into the ocean is proven to cause harm to the aquatic ecosystem and have disrupted large vegetation in seawater. The presence of pathogenic microorganisms, trace organic products, and other pollutants pose a serious threat upon long-term exposure to the environment. It has been researched that the number of contaminants in a brine could increase by 4-10 times in feedwater, thereby having a devastating effect on the ecological environment.
The prototypical characteristic of the brine varies with the quality of the feedwater, and the type of desalination process, the quality of the permeate water, the pre-treatment method and the cleaning procedures and chemicals used. These characteristics also change after pre-treatment methods and cleaning procedures as studies have shown that chemicals like acids, antiscalants and biocides have been capable of altering the chemical composition of the brine.
Profit and Extraction
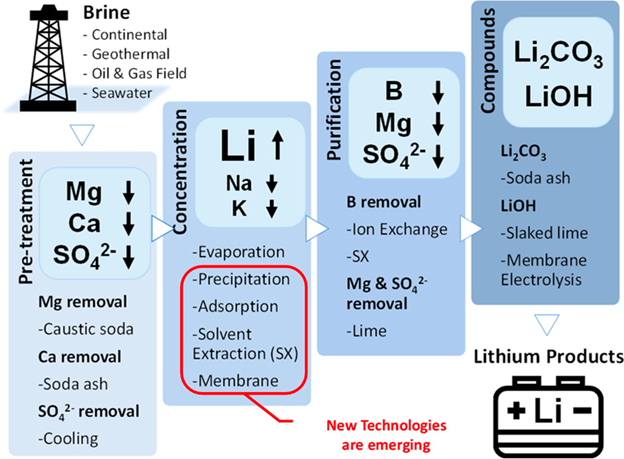
Brine has been seen a drawback of desalination for years. It was a waste product that no one knew how to properly dispose of. If it could be mined for minerals, then Brine Extraction has the potential to become an industry all on its own; because seawater contains some scarce and expensive metals, recovering metals from the brine could be an interesting objective for the water industry. To accomplish this, various types of adsorbents have been developed and used for mineral recovery from seawater and brine concentrate. An absorbent is a material which is capable of soaking up liquids in an easy and efficient manner.
Phosphates (PO43-) are precipitated using an alum blend of aluminum sulfate [Al2(SO4)3] and iron sulfate [Fe2(SO4)3]. Cesium (Cs) is recovered using a liquid-liquid extraction process by adding hydrochloric acid (HCl). Cation exchange raisins were used for the extraction of rubidium (Rb) and Germanium (Ge). Magnesium (Mg), Potassium (K), and Sodium Chloride (NaCl) are separated based on their solubility basis. An innovative method involving hexacyanoferrate-based sorbent was used to extract minerals such as Cesium (Cs), Rubidium (Rb), and Lithium (Li). Specifically, studies have shown that Lithium (Li) could be recovered from seawater using Manganese oxide (MgO) based absorbents.

However, these methods haven’t proven to be 100% effective since unwanted species get adsorbed along with target metals. Therefore, extensive research is being conducted to develop more selective adsorbents. There are currently multiple methods for brine treatment and extraction
- Forward Osmosis (FO) and Reverse Osmosis (RO)- These are one of the most emerging technologies that produces clean water using osmotic pressure difference across a membrane as a driving force and thus enables water transport from less concentrated feed stream to more concentrated draw solution. To treat brine water, one of the proposed methods involved using fructose as a draw solution for reverse osmosis (RO) brine water treatment using a cellulose acetate forward osmosis (FO) membrane. Another method proposed using ammonia (NH3) or carbon dioxide (CO2) as the draw solution with a composite forward osmosis (FO) membrane.
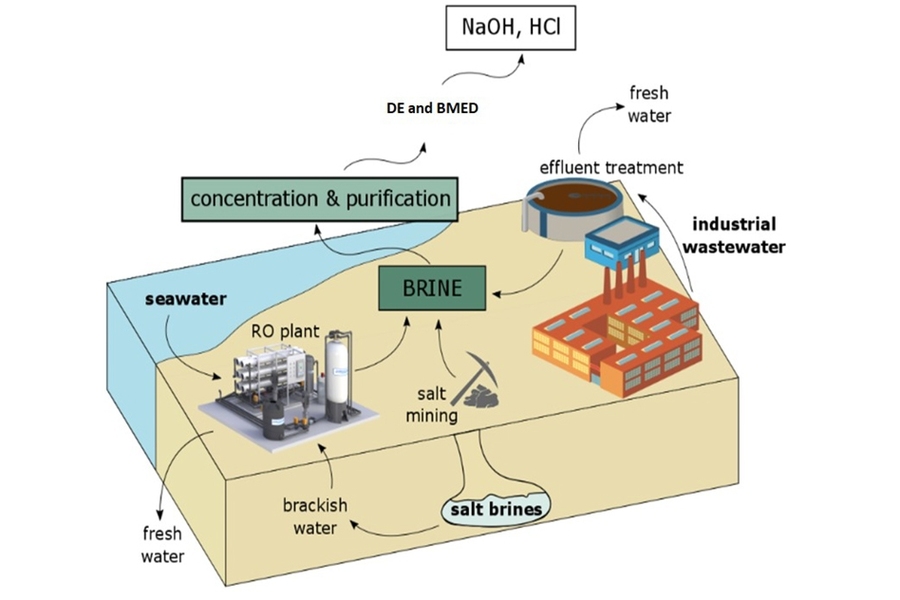
- Membrane Distillation (MD)- This method involves using hydrophobic microporous membranes for separating vapor phase from feed stream. This method has a potential use for brine management as it requires lower energy than conventional evaporation and when coupled with a solar collector system, reduces power consumption. Using membrane distillation with a crystallizer for the treatment of RO concentrate obtained feedwater recovery of 95%. Given the different components of brine solution, particularly valuable metals, this process could be explored for the recovery of resources either in the feed stream or permeate streams because of their unique transport mechanism.
- Electrodialysis (ED)- It is an electrochemical process that uses electrical currents to remove salt ions selectively through a membrane, leaving clean water behind. When researchers employed electrodialysis reversal technique for brine treatment, it promoted 92% water recovery for the treatment of RO concentrate. Also, zero discharge desalination for the treatment of seawater brines could be used for recovering salts and producing clean metal. This technology has shown to increase the brine concentration by 10 times with less energy consumption
- Hybrid Processes- This technique combines different processes to enhance water recovery of the desalination brine treatment. Integration of membrane processes could recover valuable metals together with pure water production. Hybrid process is a combination of different membrane processes like FO, MD, ED which is integrated with reverse osmosis (RO) to reap the benefits of all processes.
- Energy Recovery from membranes using emerging membrane processes- Studies have shown that using pressure reduction osmosis (PRO) technique has shown to significantly increase energy generation. Due to the salinity difference between highly saline and low saline waters, the chemical energy difference can be converted to electricity by using selective membranes, which act as semi-permeable barriers.


