Lithium
Currently, Direct lithium extraction (DLE) technology is a field where extensive research is going on. Traditional lithium extraction techniques like solar evaporation and hard rock mining have been proven to be both costly and harmful to the environment since they involve a larger carbon footprint. Seawater is the largest source of lithium in today’s world. In fact, seawater contains up to 5000 times more lithium than land. However, the problem is that seawater contains lithium at extremely low concentrations around 0.2 parts per million (ppm) compared to other elements like sodium, potassium and magnesium which are at concentrations up to 11000, 380 and 1300 ppm respectively. This leads to higher competition of these ions with lithium. Therefore, current technology makes it nearly impossible to extract lithium on a smaller scale. However, DLE technology could be implemented on a larger scale using specialized equipment.
Lithium is one of the most important elements that we know of. It has widespread applications in rechargeable batteries, mobile phones, computers, silicon chips, etc. Seawater is a major source of lithium having around 2.5*1014 kg of lithium in total which is around 0.17 mg/dm3. There are several methods for extracting lithium from seawater as listed in fig. However, the focus is going to lay on membranes for lithium extraction. Over recent years, membrane technology has possessed the highest capability of extraction of lithium from seawater itself. Membrane-type adsorbents and polymeric membrane reservoirs are two ways which have been scientifically proven to be the most effective in extracting lithium from seawater. Adsorption is simply defined to be the gathering of individual molecules, atoms or ions on specific boundary surfaces. The former method involves the use of spinel-type manganese oxide (MnO2) as the membrane-type adsorbent prepared by solvent-exchange method using poly-vinyl chloride (PVC) while the latter method involves preparing a polymeric membrane reservoir for the recovery of lithium from seawater.
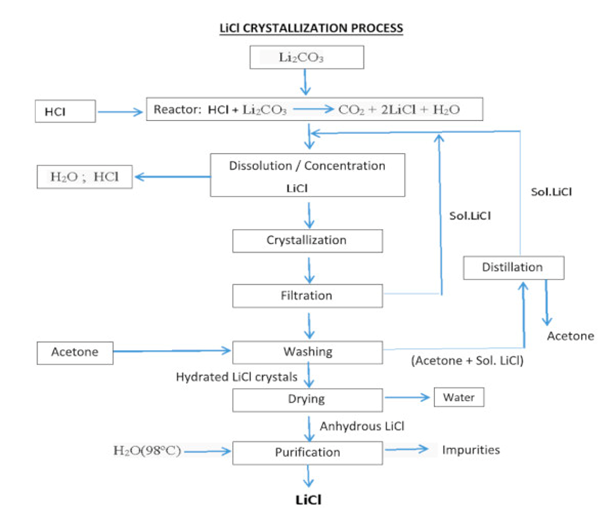
Typically, lithium extraction undergoes multiple steps using different industrial equipment. Firstly, filtration machines are used to remove all the initial impurities like seashells which may be present in the brine and then make this brine is made to react with adsorbent materials in a reactor to remove lithium through the process of adsorption. Adsorption is simply defined to be the gathering of individual molecules, atoms, or ions on specific boundary surfaces. It is the separation of a substance from one phase by its accumulation at the surface of another phase. The adsorbing phase is called the adsorbent, whereas the material concentrated at the surface of that phase is called an adsorbate. This reaction of the brine with an adsorbent usually happens at high temperatures. The extraction process is continued by sending the lithium onto the loading and washing reactors to wash any residual brine off the solid adsorbent material. This material is then moved as a slurry to the stripping reactor where the slurry is contacted with dilute hydrochloric acid (HCl) to make a concentrated lithium chloride (LiCl) solution which could then be purified using ion-exchange devices to obtain pure LiCl. Lab results indicate that quality grade LiCl solution is produced using this process with a purity of greater than 90%.
These proposed methods for lithium extraction are obtaining lithium directly from seawater instead of using brine. The former method mentioned in the previous paragraph is designed specifically for natural seawater flow having a lower hydraulic pressure where membranes are set parallel to the cell and seawater can flow smoothly through the membranes. Lithium in brine coexists with alkali metals like magnesium and alkali earth metal ions. Because of similar chemical properties between lithium and magnesium, this increase difficulties of lithium salt separation and extraction. However, some methods like electrochemical extraction and precipitation could be used to extract lithium from brine. These are new promising technologies in the field of lithium extraction.
- Electrolysis- Involves the use of two types of electrodes which are cathode and anode. The mechanism of extraction is based on these two electrode materials – one of which acts as a Li-insertion material, whereas the other is used to capture the corresponding negative ions. Some electrode materials for advanced lithium-ion extraction have been developed and proposed. These are lithium iron phosphate (LiFePO4) and lithium manganate (LiMn2O4) which act as Li insertion material. LiMn2O4 is more suitable due to its higher stability. These compounds have advantages of lower power consumption and lower prices. However, since Li and Mg have properties which are like each other, it is difficult to separate them from brine. Alternatively, LiFePO4 and FePO4 compounds could be used to extract lithium as it has good reversibility in LiFePO4/FePO4 solution. LiFePO4/Ag could be used to efficiently recover lithium from brine. LiFePO4 is the typical lithium capture carbon electrode. Furthermore, the MnO2/Ag system also successfully demonstrated consumption of 1.0 W h per recovery of 1 mole lithium in simulated brine.

- Other cathode/anode combinations included the use of 𝞪-MnO2/ Ag with 𝞪-MnO2 acting as a positive electrode and Ag acting as the anode. This electrode combination overcame the limitation of Mg ion interference and demonstrated consumption of 1.0 W h per recovery of 1 mole lithium in simulated brine. Moreover, the given combination has a higher cycling stability compared to the previous combinations. Cycling stability is measured as the number of charging and discharging cycles of the electrodes before which its capacity is reduced to a certain amount of its nominal capacity. Also, LiFePO4/Zn have been used instead of using Ag to promote cost-effective resources for lithium extraction. The system consists of LiFePO4 which is used as a positive electrode and zinc is used as a negative electrode along with an anode exchange membrane.
- Precipitation- Has been one of the earliest technologies which has been studied and implemented in industrial plants for lithium extraction from Salt Lake brines. It is the process of transforming a dissolved substance into an insoluble solid from a supersaturated solution. The solid formed from this process is called a precipitate. The major advantage of this technique lies in the cost-effectiveness and simplicity. Lithium could be separated from brines containing high amounts of magnesium leading to a high Mg/Li ratio, by magnesium and illuminate precipitation methods. These methods promote recovery of lithium and magnesium resources as products. In this manner, the brine is comprehensively utilized. Precipitation could be carried out by firstly concentrating the brine by solar evaporation and increasing the lithium concentration up to 6000 ppm. Sodium (Na+) and potassium ions (Na+ and K+) can be precipitated above their saturation point. Magnesium, calcium and sulfate ions (Mg2+, Ca2+ and SO42-) can be precipitated using lime calcium hydroxide (Ca (OH)2), Sodium carbonate (Na2CO3), Oxalate (C2O42-) and Barium Chloride (BaCl2). The process can be divided into carbonate precipitation, aluminate precipitation, carbonization precipitation, Boron (B) and Lithium cooperation.

- The process is initiated by first precipitating the various coexisting ions other than lithium (Mg2+, Ca2+, SO42- and B3+) to obtain a lithium rich solution. High-purity lithium carbonate products can then be obtained by further treatment. Boron could be separated using adsorption in a low pH solution (typically less than 11.3) after magnesium and calcium are removed by precipitation using (Ca (OH)2). After obtaining a high lithium concentration of up to 2g/L by evaporation of brine, further impurity purification is done using Na2CO3 at 80-90oC. The enriched lithium could then be precipitated in the form of lithium carbonate (Li2CO3) with a 99% purity. Following this, lithium could be obtained from Li2CO3 using electrolytic separation. This process obtained high lithium extraction and Mg2+ precipitation rates of 99% and 86.5% respectively. Typically, a low pH solution is desirable

