Construction
LET’S TALK ABOUT CONCRETE
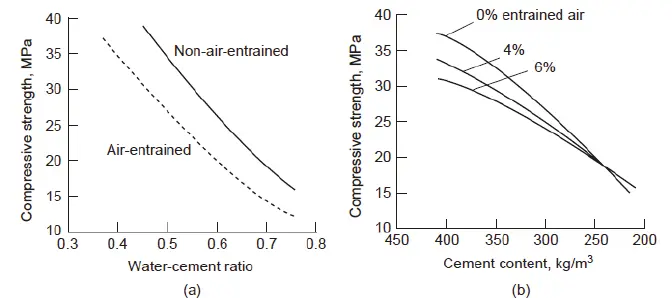
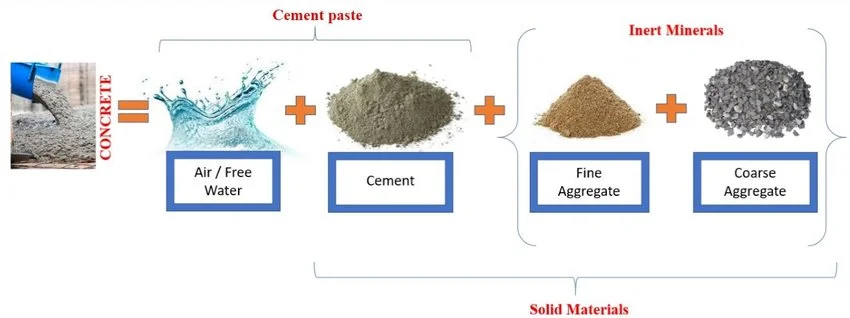
Concrete is a composite material consisting of hard, chemically inert particulate substances known as aggregate. Aggregate means to combine or mix together. Some examples of aggregates used in concrete are sand, gravel and water. Aggregates in science are majorly defined to be fine (small) or coarse (large). These are usually free from soft particles and organic soil compounds. Concrete has a widespread application in construction industries to make structures due to their hardness and durability. It is bonded together by cement and water and is characterized by the type of aggregate or cement used. The character of concrete is largely determined by the water to cement ratio. It has been proven that the smaller the water content, the stronger the concrete. Decreasing water quantity improves density of the paste and increases watertightness, which in turn increases durability and resistance to chemical scaling and water attack. Moreover, the quality of water used (river water, well water, rainwater, etc.) also affects the quality and strength of concrete. Usually, sand particles present in water bodies are the major source of producing concrete. Research suggests that more than 90% of the sand in water bodies have been used in the construction industry, with over 45% of the dredged sea sand used as fine aggregate for concrete. Desalted sea sand has been used as one of the major sources of fine aggregate for concrete production in countries like Japan, UK, and Netherlands.
Concrete is a composite material consisting of hard, chemically inert particulate substances known as aggregate. Aggregate means to combine or mix together. Some examples of aggregates used in concrete are sand, gravel and water. Aggregates in science are majorly defined to be fine (small) or coarse (large). These are usually free from soft particles and organic soil compounds. Concrete has a widespread application in construction industries to make structures due to their hardness and durability. It is bonded together by cement and water and is characterized by the type of aggregate or cement used. The character of concrete is largely determined by the water to cement ratio. It has been proven that the smaller the water content, the stronger the concrete. Decreasing water quantity improves density of the paste and increases watertightness, which in turn increases durability and resistance to chemical scaling and water attack. Moreover, the quality of water used (river water, well water, rainwater, etc.) also affects the quality and strength of concrete. Usually, sand particles present in water bodies are the major source of producing concrete. Research suggests that more than 90% of the sand in water bodies have been used in the construction industry, with over 45% of the dredged sea sand used as fine aggregate for concrete. Desalted sea sand has been used as one of the major sources of fine aggregate for concrete production in countries like Japan, UK, and Netherlands.
The figure shows the primary composition of concrete. The water-cement ratio is usually around 0.5 in a typical concrete and increases as the strength of concrete decreases.
WHAT IS THE COMPOSITION AND CHARACTERICS OF SEA SAND AND SEAWATER CONCRETE?
In general, sea sand needs to be properly processed before being used as concrete to avoid steel corrosion problems in reinforced steel structures. Therefore, standards to enforce seasand desalting need to be taken before using sea sand for concrete construction. The use of sea sand without appropriate desalting has led to failures around the world like collapsing of seasand houses in countries like South Korea, Turkey, and China. Techniques like X-ray diffraction (XRD) and petrographic analysis have shown that sea sand has similar mineral composition and geological properties like river sand but differ in surface textures, which may affect the interlocking properties and strength of the concrete. The particle size distributions of sea sands obtained from different countries are within or near the limits of Grade II Sea sand (probably a particular type of sea sand used for construction) and is therefore appropriate for the use of concrete construction. Moreover, sand extraction location also needs to be considered to determine and pinpoint the exact properties of sea sand. Sea sand contains more salt than river sand, as well as seashell particles and potentially harmful substances which need to be removed before their application in aggregates used in concrete. Sea sand has higher density than river sand due to the chemical composition of seashell particles containing CaCO3.
The figure portrays the Compressive strength (in MPa) graph of sea sand compared to river sand over a period of 28 days with different composition of water
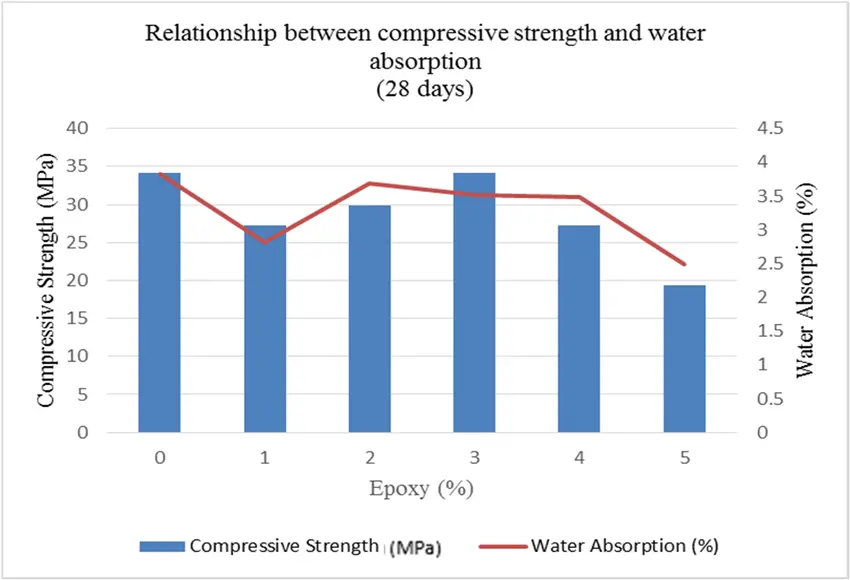
Generally, it has been proven that limited salt in water will supply a gain in strength of concrete. Sea sand is usually used to make concrete instead of seawater since a small percentage of impurities in sea sand like coal, chalk or clay is unlikely to affect the workability of concrete. The compressive strength (maximum stress that a solid can sustain without any fracture) of sea sand concrete is approximately the same as ordinary concrete at the initial stage. However, significant loss of compressive strength was observed when 50% of the ordinary fine aggregate was replaced with seashell particles. Most studies have shown that seawater concrete may have a higher early-age strength compared to ordinary concrete, but the long-term compressive strength of seawater concrete is inferior to ordinary concrete. The durability of concrete generally depends on chloride (Cl- ) and sulfate (SO4 2-) diffusion, carbonation behavior, freeze-thaw resistance as well as drying shrinkage and creep. Creep is the time dependent deformation under a certain applied load. Chloride content of greater than 0.5% caused a decrease in freeze-thaw resistance and durability of the concrete. However, steel reinforcement corrosion became significant when the concentration of free Cl- in sea sand exceeded 0.3%. This is because chlorine is the best oxidizing agent and thereby highly corrosive in nature.
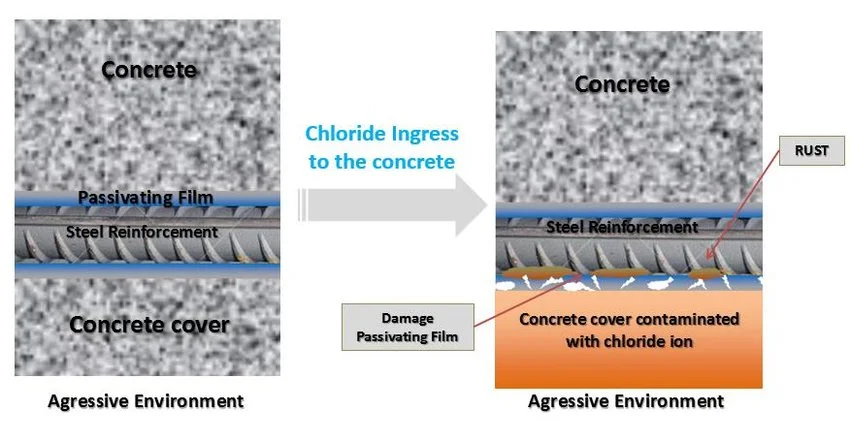
A representation of the deteriorating effect of chloride ingress on concrete. The presence of chloride ions in sea sand could strongly corrode steel
WHAT SHOULD WE DO TO INCREASE THE CORROSION RESISTANCE OF STEEL TO SEA-SAND HAVING HIGHER CHLORINE CONTENT?
Mineral admixtures such as metakaolin and fly ash improved chloride resistance of concrete by increasing the compressive strength of concrete up to 52%. Also, fiber enhanced composites (FRPs) like aramid FRP, basalt FRP, glass FRP (GFRP) and carbon FRP are common types of FRP’s which could be used to protect steel from the chloride content in seawater or sea sand. Also, other materials that could be used to provide chloride resistance are epoxy-coated steel bars, stainless steel bars and carbon fiber rods. FRP rebars (a bar used for concrete construction) could be used as a replacement of steel rebars to enhance the mechanical characteristics and provide durability of concrete. Since FRP’s are made up of continuous fibers impregnated in a polymer resin matrix, the resin binds the fibers together, transferring stresses between them and protecting them from environmental attacks. GFRP is the most popular material for concrete structures due to excellent electro chemical corrosion resistance and relatively low cost compared to other forms of FRPs. Thereby, many projects involving FRP-sea-sand concrete structures have been effectively proposed to mitigate sea sand corrosion. In fact, the first FRP bar-reinforced sea sand structures were proposed for marine infrastructure in the year 2011 in Hangzhou, China. Lastly, since the construction industry has been long criticized for its massive environmental pollution, the use of recycled aggregate concrete (RAC) with sea sand and seawater has been proven to have high mechanical and compressive strength along with reducing carbon footprint. Moreover, the direct use of RAC is no longer a concern for FRP sea sand structures.
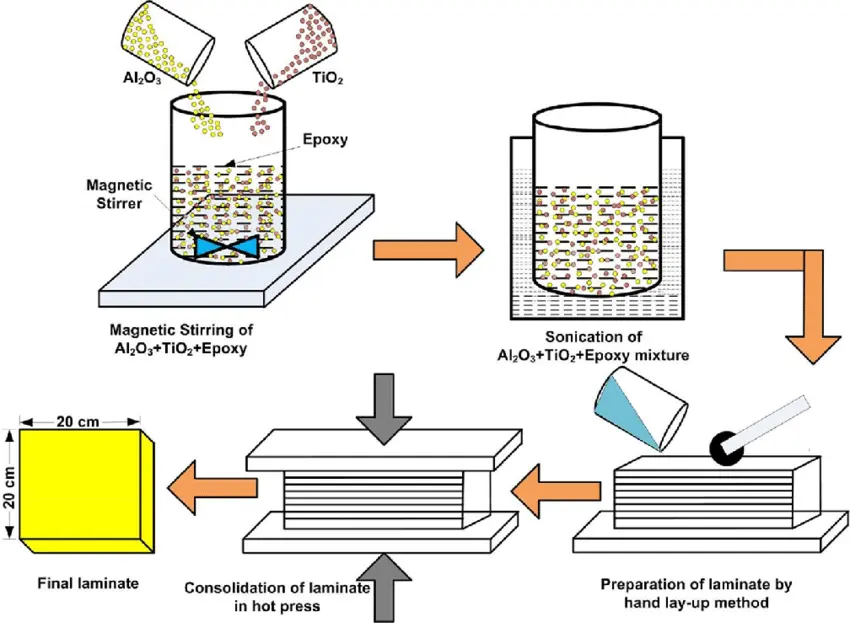
The figure depicts the application of Glass fiber enhanced composites (GRFPs)
reinforcement in concrete construction
HOW DO WE SEPARATE SEA SAND FROM SEAWATER FOR MAKING CONCRETE?
The simplest way to separate sea sand from seawater is by using the solubility difference between the two elements. Salt is soluble in water and forms a homogeneous (uniform) mixture whereas sand is insoluble in water and forms a heterogeneous (non-uniform) mixture. Thereby, we can first completely dissolve the salt in seawater by adding some freshwater and stir it for some time. If salt still isn’t dissolved, we can heat it on gas in a pan for a little while (2-3 minutes) to promote efficient solubility. Following this, we can filter the seawater through a funnel using a simple coffee filter into the beaker. This should enable the salt water to pass through the filter and end up in the beaker, leaving the sea sand behind on the coffee filter. This sea sand could then be used as a fine aggregate for concrete construction. If we want to reobtain the salt/brine solution, we can simply put the salt solution back into the pan and heat it until all the water evaporates and brine is left behind.
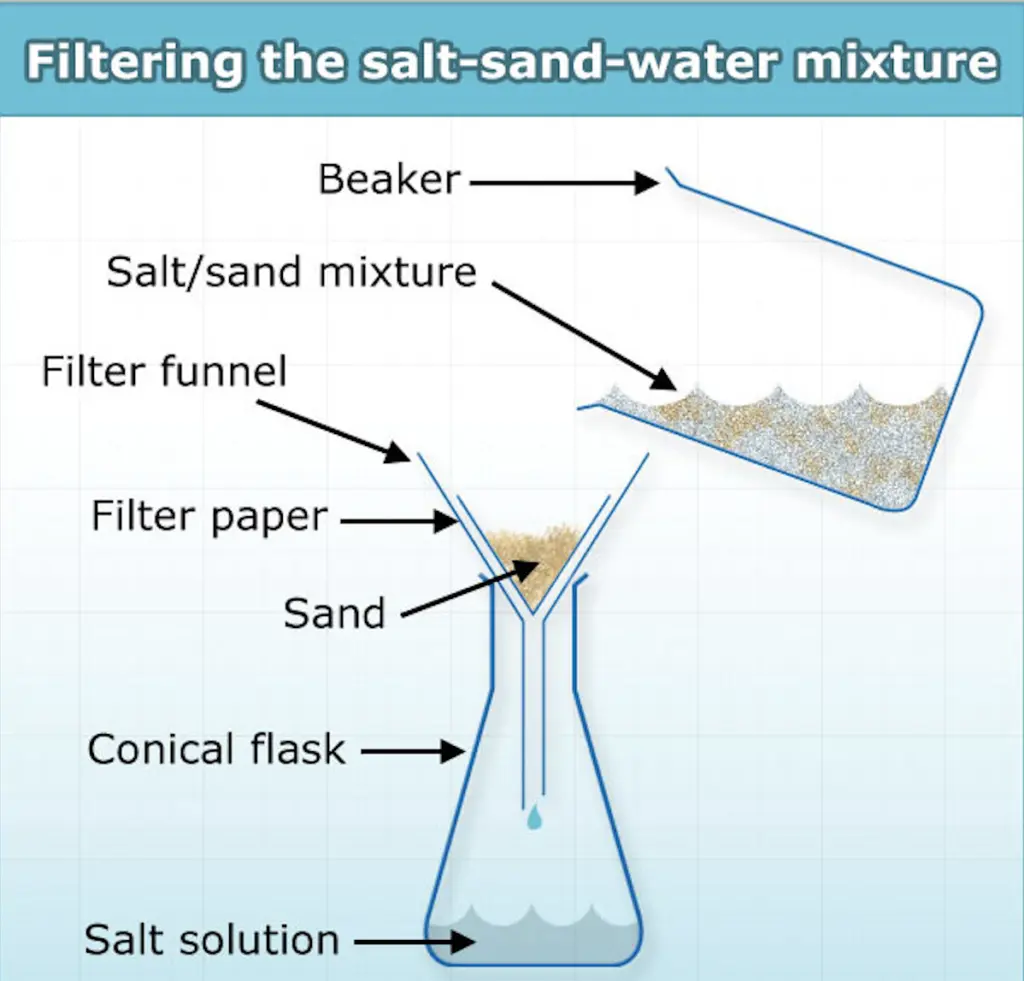
The figure outlines the separation process of sea salt and sea sand using a fiber placed in a funnel
HOW DO WE EXACTLY MAKE CONCRETE FROM SEA SAND AND SEAWATER?
In one study conducted by researchers in Japan, a mixture of seawater and tap water was used for mixing. Before using seawater, OPC (Ordinary Portland Cement), GGBS (GroundGranulated Blast-Furnace slug), FA (Fly Ash) and SF (Silica Fume) were used as binders. Binders are materials that bind chemically and mechanically to impart structural support and mechanical strength to the cement. GGBS and FA can considerably reduce CO2 emissions due to the reduced consumption of cement. CO2 emissions from constructions using sea sand and seawater concrete (SWSS) were 40% smaller than those constructions which used freshwater and land sand concrete. The water-binder ratio was kept at 0.5 and the fine aggregate (land sand in our case)-binder was kept at 3.0. Land sand (S) and crushed stones (G) were used as fine aggregates and coarse aggregates respectively. Before using land sand, it could be made to pass through a 2 mm mesh sieve to remove sharp shell fragments, grasses, and a few organic materials. Water reducer (AE) and calcium nitrate (CN) were used as admixtures. Concrete admixtures are natural or manufactured chemicals or additives that are added to concrete during concrete mixing to enhance the specific properties like workability, durability etc. of the fresh and hardened concrete. Different permutations and combinations involving water type, calcium nitrate presence, OPC, GGBS, FA were done to determine the combination with the best compressive strength, water permeability, freeze-thaw resistance, and corrosion resistance of reinforcement for concrete construction. This is shown below. These combinations are shown below in figure N.
The figure shows the different compositions of various materials used in concrete. The main materials used are water, binder, aggregates, and admixtures. These materials have several subcategories and the permutation of these subcategories of materials are presented in the given table
After testing all the given combinations of mortar, one particular combination involving the use of seawater and calcium nitrate (CN) showed higher values of compressive strength. In general, when GGBS and FA are used as binders, the use of sea water and addition of CN and SF are found to significantly increase the early strength and moderately increase the longterm strength of the concrete. Addition of CN to concrete increases the OH- ion concentration in the pore solution and intensifies the alkali stimulus to GGBS and FA, thereby accelerating hydration and increasing compressive strength. Also, the permeability coefficient of concrete is evaluated to determine the watertightness of concrete. We want less permeability and more watertightness for the concrete to bind strongly. Results plotted on the graph shown below indicates that permeability coefficient using seawater and seawater+CN/seawater+CN+SF is significantly lower than the permeability coefficient of concrete mixed with tap water, thereby indicating that seawater+CN significantly improves watertightness of concrete.
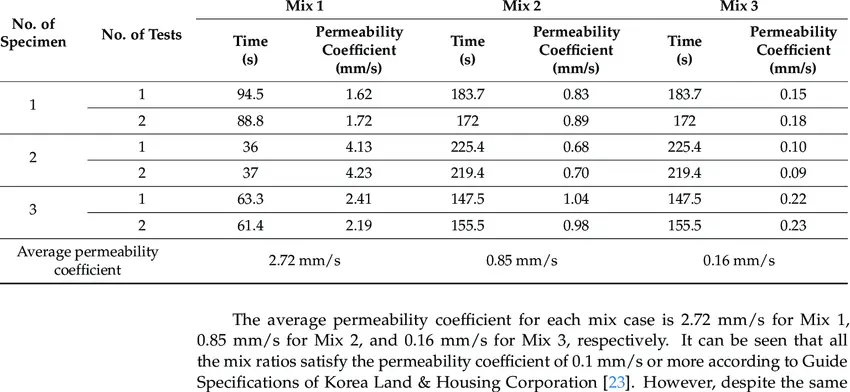
The figure shows the permeability coefficient of concrete in m/s for various types of water used in concrete. Seawater with calcium nitrate and silica fume shows the least permeability and thereby the highest watertightness for concrete strengthening
For freezing and thawing cycles, it was shown that SWSS having an air content of 3.5% were adequately resistant to freezing and thawing actions. Other tests were also conducted on the concrete length change ratio due to drying shrinkage strain of SWSS concrete which was smaller compared to tap water concrete. Following this, researchers also tested the concrete for corrosion and deterioration reinforcement using epoxy-coated steel reinforcement, carbon fiber rods and normal steel reinforcement. At the end of 33 cycles corresponding to 100 years in a marine environment, the surfaces of normal steel bars were completely corroded due to the presence of Cl- ions in SWSS concrete. However, no corrosion or deterioration was observed in epoxy-coated steel bars or carbon fiber rods. Therefore, corrosion resistant reinforcements like stainless-steel bars, galvanized steel bars, or carbon fiber bars as mentioned above should be used for concrete reinforcement. Also, metal materials such as separators and embedded metal fittings should preferably be coated with a corrosion-resistant material before being used in concrete or concrete reinforcement.
BRINE
Brine in the UAE
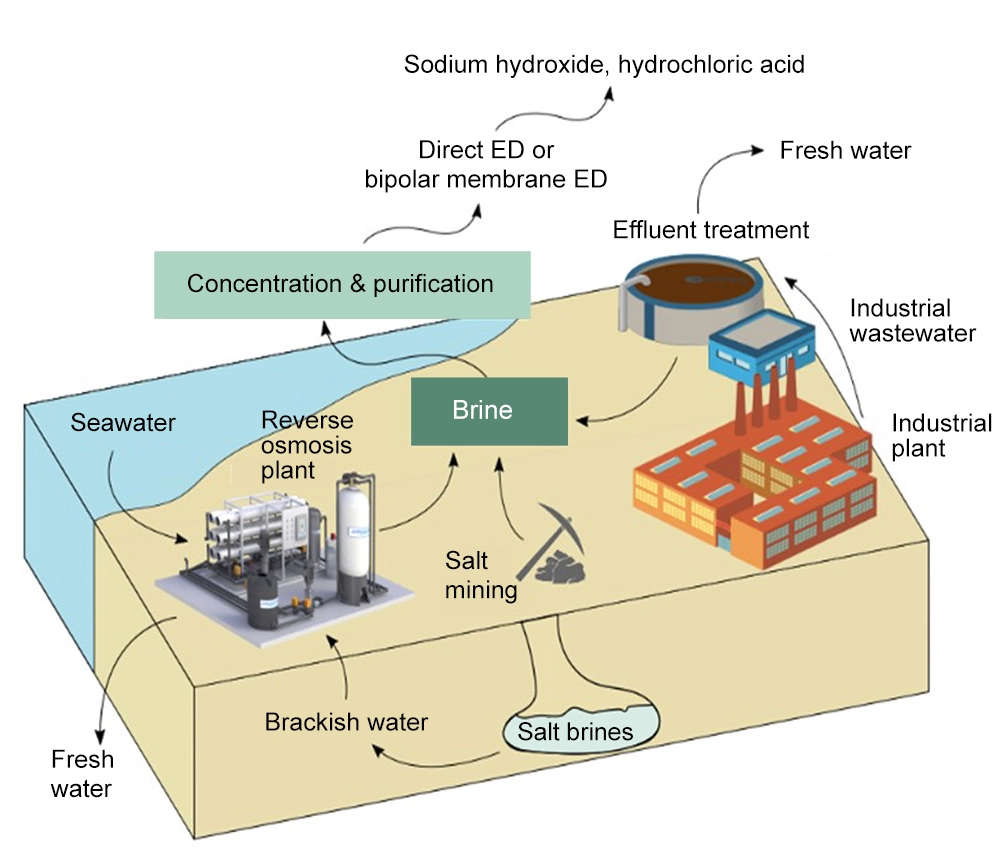
In the UAE, they produce nearly 28 million cubic meters of brine daily, so they have made it a huge focus on a national level to repurpose it. There was an investigation conducted to see the methods of brine disposal, specifically at 8 RO plants and in the insides of Oman. Currently, they dispose of brine in pods, oceans, beaches and “unlined small bores” but this can be problematic as if the brine reaches other elements in the groundwater, such as iron, copper, and zinc, if the brine there can be serious implications. Therefore, studies to see different manners of repurposing brine to reduce the possible consequences of desalination have been conducted. One way they have thought of is to use it in place of salt to create blocks that can then create architecture. In the past, Sabkha was used, which were blocks made from salt that can be used to build entire towns. Siwa in Egypt is one example from the past of this. The goal is to reconstitute this idea but with brine as the material rather than the salt flats of their wetlands.
Brine As a Fuel?
Hydrogen and Chlorine
There is a serious possibility by replacing photocleavage of water with that of brine. Water splitting “requires the transfer of four electrons per oxygen molecule formed” so this is difficulty to do eve while using extremely powerful water oxidation catalysts. Therefore, its efficient use is extremely difficult while it is the most prominent method currently. How brine can instead be used is that it would only require 2 electrons thus require much lesser electric potential. In doing this, brine would be converted to hydrogen and chlorine gas, which can be used as a fuel sources. However, chlorine gas would be hard to transport due to expense and safety so a means to make it portable would be necessary. Regardless both can be very useful. They also provide two oxidized chloride products which have various other uses, which are NaOCl or chlorine gas. The uses of NaOCl are for bleaching, water disinfecting, odor removal, and surface purification and the uses of chlorine gas are for bleach and pesticides primarily. So, a byproduct of brine can have many uses amongst being used as a fuel source primarily. This would be via hydrogen as this can be “converted to electricity via a fuel cell” or “stored and used as a fuel”.
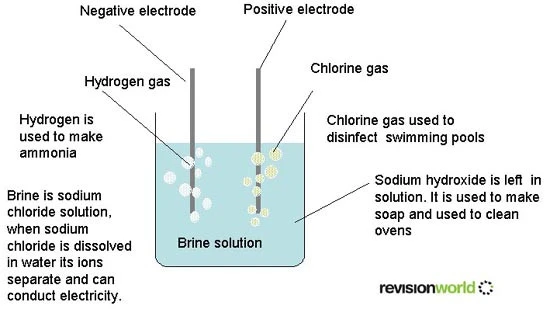
The figure shows how brine has various uses for both hydrogen and chlorine gas because it can produce sodium hydroxide and hydrogen gas, which are extremely useful. It is a conductor of electricity as the diagram above shows and therefore there are attempts to use it as a fuel source. Current other uses can be as a disinfectant, as it can be used as a pool disinfectant, ammonia, and soap solution.
Electrodialysis
The process of doing so would be via reverse electrodialysis as it would serve to generate electricity. It would work by using the water with high salinity, such as brackish water, as the dilute as you can maximize the power by reducing electrical resistance with its use. There is still many variables that must be understood and therefore a lot more research to be conducted. One project that will be focusing on this heavily is the REAPower project. To dive into more of the scientific process, the membrane of the brine must be stable and robust so that it can deal with the “chemically aggressive electrolyte” and “have a low electrical resistance” so that it can be easily electrolyzed. It must be a producer of NA+ and its membrane be “impermeable to Cl- and OH-, which is common in “perfluoro-sulfonic acid membranes” and therefore this would be the goal for the Brine membranes to emulate. This membrane is one that produces polymers as stated prior. This is necessary so that it can successfully separate products from the reaction and transfer ions between the electrodes and therefore create an electric charge.
The figure shows the process of electrolysis in detail. This is the process through which brine would be used as a fuel source as the properties of brine would allow it to act as a conductor of electricity.
Texas
Texas attempts to use brine as an energy source as well. The company, Texas Brine, is working with Mitsubishi Power to do so, by transforming the underground salt caverns that lie in Texas into “storage sites for green hydrogen”. The method that they would go about this is to extract brine from chambers with salt and water so that there is space for hydrogen storage for large gaps of time. This hydrogen can then be used as s clean fuel source. This however does not address the issue of repurposing brine as a means of a new fuel source and there is not much on how the brine will be used. From other sources, it is noted that brine can be used for roads during poor conditions, and heavily in the chlor-alkali industry. Specifically, it is used to cover roads to decrease the probability of the roads freezing.
Brine Disposal
There is a goal of treating brine through the energy of the sun as current methods of disposal are not environmentally friendly. The current methods are to use evaporation ponds and to dump it back into the ocean. How this process would work is through an aluminum separator. It would conduct heat at the walls of the aluminum, and this would work to allow for evaporation. This process would only work on a smaller scale however and this would therefore be a disadvantage of this process.
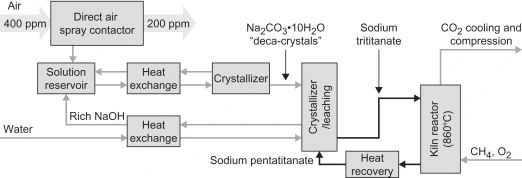
The figure shows the process of disposing of brine in a more environmentally friend manner through a solar crystallizer made of aluminum.
Brine and the Photovoltaic Effect
The intent with the use of brine is to increase the efficiency of photovoltaic panels by cooling them and to increase the efficiency of “RO systems by preheating the feedwater.” The method of doing so is being determined via tests which have shown that brine rejection decreases when the feedwater temperature rises, and this causes for a faster recovery. Then for the cooling of the panels, there is a more significant cooling when brine is used, if it is at or less than the ambient temperature. There was a maximum rise of 3.3 percent rise in electrical efficiency for the panels at cooling conditions; this is 20 percent higher than noncooling conditions. It also has another great economic benefit as it shows that for RO systems using brine rejected directly from these systems rather than water it can reduce the payback period by 2-4 years.
Another intent of it is to reduce the waste brine to zero in the process of desalination and cooling the panels. How this would work is that the excess heat would be directly used for water evaporation through “multistage membrane distillation component” located on the back of the solar cell. The waste brine will be converted into a crystallized salt solid and there will be no liquid discharged. This crystallized salt solid can be used in the ways mentioned prior, particularly in Texas.
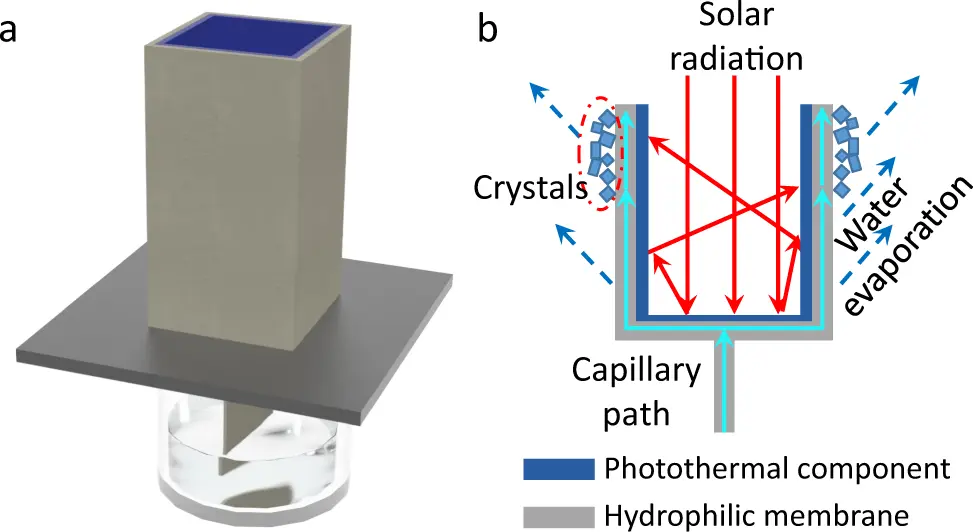
The figure shows a process through which the brine waste is converted to crystal solids with not waste as a byproduct.
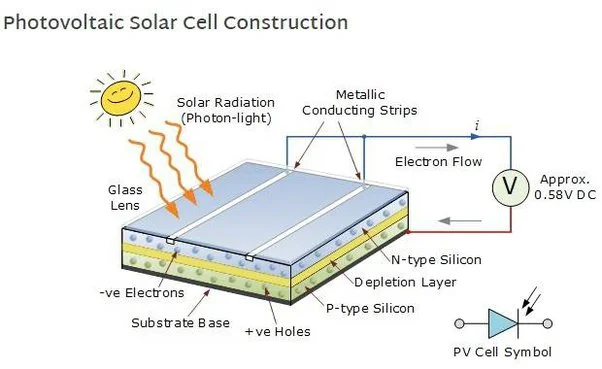
The figure demonstrates how an electric current is generated via the photovoltaic effect. The electrons are holding the semiconducting material together when they are not excited as they form bonds with nearby atoms. However, when they are in the excited state they are free to move so they move to the opposite direction at first and then move to the n-side rather than the p-side, creating “an electric current in the cell.”
Saudi Arabia
Over 3 million cubic meters of drinking water produced daily through 27 desalination stations by the Saline Water Conversion Corporation. This is a large portion of their water used as it accounts for 70 percent of the water of the cities in Saudi Arabia.
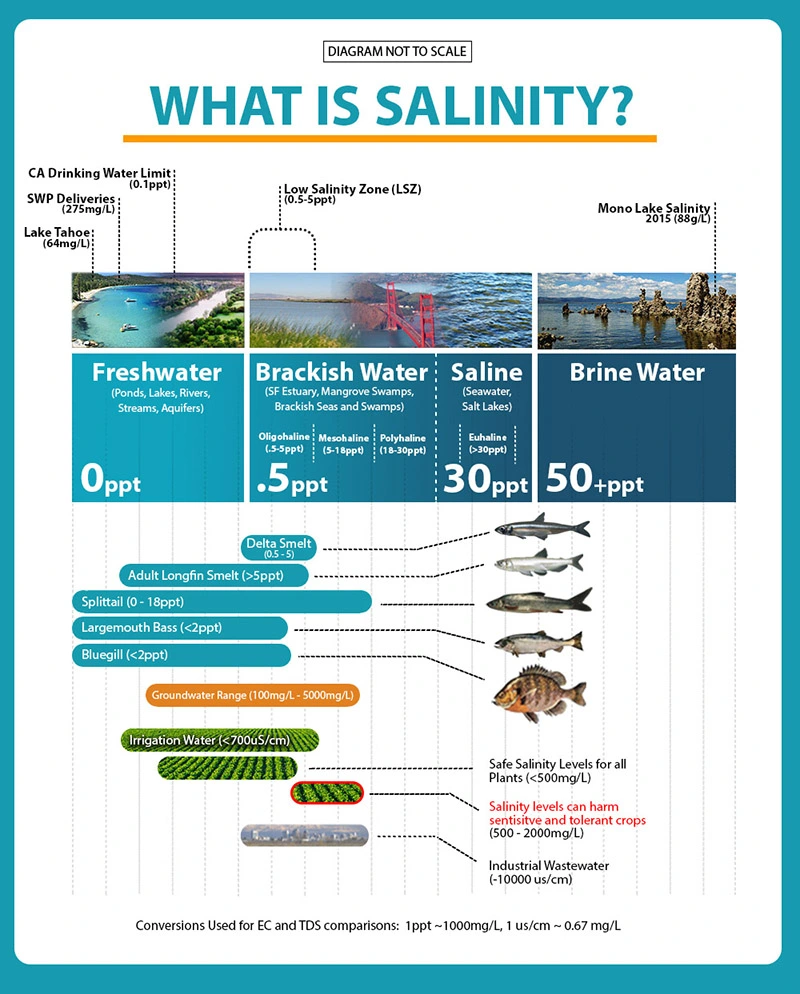
The figure shows how different types of water have different levels of salinity and thus have different concentrations of brine, since brine is “a high concentration solution of salt in water”. Fresh water has a very small percentage of salinity as we expect it to. Whereas seas and more swampish areas have a much higher concentration of salinity. The Red Sea in particularly seems to have a percentage of salinity and this is important to note as it is in the Arabian Peninsula. This can be indicative of part of the reason why there is such a large amount of brine in Saudi Arabia.
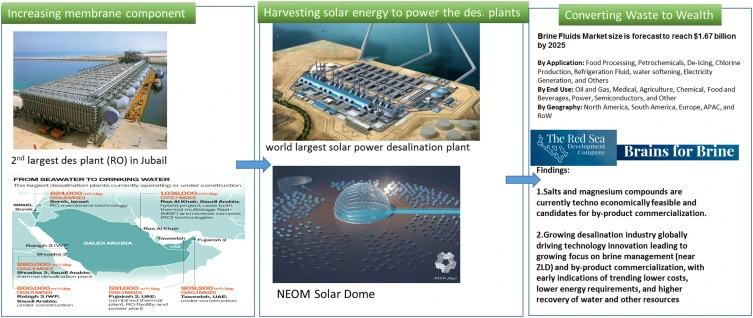
Figure 20 Shows how Saudi Arabia creates a large amount of brine output as they have a highwater scarcity as can be seen. Has seven projects geared towards reducing this issue, even here can use brine to remove chemicals along with heavy metals. But this is very expensive and requires a lot of energy. This issue is further accentuated because near the Arabian Gulf there is an increase of 0.02 g/kg increase in saline over the span from 2015-2020. Albeit this may not seem like much it really has become very dangerous for organisms living in that area.
Concrete mixed with Brine
The idea between this idea is to create a mixture of cement and brine to create concrete. Different amount of brine can be used but the article suggests 50 percent. This would in turn increase the strength of the concrete by 16.5% which is a large increase. This can have many implications as it allows architecture to a different level as the strength of an important material increases.
Brine in Other Uses
Brine can also be used for preservatives for meat packing and for pickling as it has a very low feeding point and thus is great as a method of heat transfer. Likewise, it absorbs vapor very well as it has a lower vapor pressure. Therefore, it has many other uses in the food industry. It also has further uses as it cools steal as well.
